Repurposing of Potential Drugs - Potential Novel COVID-19 Medicine
Existing antivirals and knowledge gained from the SARS and MERS outbreaks gain traction as the fastest route to fight the current coronavirus epidemic.
Repurposing of Potential Drugs: A Hope to Combat Global Novel COVID-19 Outbreak
Highlights
- Remdesivir inhibits SARS-CoV-2 life cycle
- Chloroquine inhibits SARS-CoV-2 entry
Global outbreak of SARS-CoV2
Global outbreak of SARS-CoV2 with an uncertain fatality rate has imposed extreme challenges on global health. The World Health Organization (WHO) has officially declared the outbreak of COVID-19 a pandemic, after the disease caused by the new coronavirus spread to more than 100 countries and led to tens to lacs of cases within a few months (May 2, 2020). Lethal zoonotic SARS-CoV have caused a global outbreak with fatality rate 10% in 2003 and MERS-CoV had imposed infection globally with 35% fatality rate (as of November 2017). These coronaviruses are associated with novel respiratory syndromes. Highly contagious background of SARS-CoV-2 is making it pandemic.
No effective drug or vaccine has yet been approved to treat 2019-novel coronavirus. Medicines for the treatment of 2019-novel coronavirus (COVID-19) infections are urgently needed. Advances in the study of highly pathogenic coronaviruses reveals the resemblance of COVID-19 with severe acute respiratory syndrome (SARS) & Middle east respiratory syndrome (MERS). Like SARS and MERS, the 2019-nCoV is an enveloped, positive sense, single-stranded RNA beta-coronavirus. The 2019-nCoV genome encodes non-structural proteins. RNA-dependent RNA polymerase, helicase, 3-chymotrypsin-like protease and papain-like proteases are the non-structural proteins encoded by 2019-nCoV genome which is showing more than 90% sequence of essential enzymes present on genome of SARS-CoV are similar to genome sequence of SARS-CoV-2. Accessory proteins such as spike glycoprotein are structural protein also been observed on 2019-nCoV genome. Antivirals agents against SARS and MERS were developed by recognizing these five proteins as attractive targets.
Approaches that inflect crucial host elements of viral infection, neutralize structural proteins and antagonize viral nonstructural proteins are the one significant way to develop drug and vaccine for COVID-19. In the interim, small molecule drugs, peptides, oligonucleotide-based therapies, monoclonal antibodies, interferon therapies and vaccines can be envisaged to control or leads to outbreak of COVID-19 to an end. The potential to repurpose existing antiviral agents approved for treating infections caused by HIV, hepatitis B virus (HBV), hepatitis C virus (HCV) and influenza, based on therapeutic experience with two other infections caused by human coronavirus (SARS and MERS).
Antiviral compounds against SARS-CoV-2
Antiviral compounds previously reported to show effect against SARS-CoV or other coronaviruses may be effective against SARS-CoV-2. Several drug candidates including ribavirin, lopinavir-ritonavir, and favipiravir, have been used previously to treat SARS or MERS, and these compounds may have potential in treating patients with SARS-CoV-2 from this current outbreak. One study by Xiao et.al has evaluated chloroquine, nitazoxanide, ribavirin, remdesivir (GS5734), nafamostat (Protease inhibitor) and favipiravir (T-705) for antiviral efficiencies. A series of clinical trials has been started on account of COVID-19 outbreak. Clinical trials of remdesivir, vitamin C infusion, hydroxychloroquine, ritonavir-lopinavir combination, cobicistat and darunavir has come up with significant dimension on COVID-19 horizon. Given no specific antiviral therapy for COVID-19 and the availability of remdesivir as a potential antiviral agent based on pre-clinical studies in SARS-CoV and MERS-CoV infections, this randomized, controlled, double blind trial will evaluate the efficacy and safety of remdesivir in patients hospitalized with mild or moderate COVID-19.
Remdesivir (GS-5734): Adenosine analogue
An experimental antiviral drug Remdesivir, made by the Bay Area's Gilead Sciences has found to show in vitro activity against SARS-CoV-2.
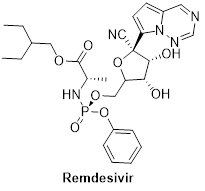
Remdesivir mimics nucleotide (ATP), added during RNA synthesis and it binds to nucleotide binding pocket of RNA dependent RNA polymerase (RdRp). Hence, it inhibits the synthesis of new RNA strand by RdRp. This terminates corona virus genome replication. Remdesivir, reportedly had a successful clinical trial in Chicago and has been putting on clinical trials by other coronavirus infected countries.
Gilead has initiated two Phase 3 clinical studies to evaluate the safety and efficacy of remdesivir in adults diagnosed with COVID-19 following the U.S. Food and Drug Administration’s (FDA) rapid review and acceptance of Gilead’s investigational new drug (IND) filing. These randomized, open-label, multicenter studies began enrolling patients in March 2020 and will enroll a total of approximately 1,000 patients in the initial phase of the studies, in countries with high prevalence of COVID-19. FDA issued an emergency use authorization for the investigational antiviral drug remdesivir for the treatment of suspected or laboratory-confirmed COVID-19 in adults and children hospitalized with severe disease.
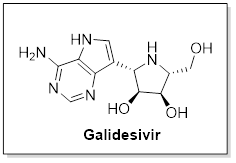
Immucillin-A (galidesivir), an adenosine analogue
Galidesivir, broad spectrum RdRp inhibitor against several RNA viruses, such as paramyxoviruses, flaviviruses, togaviruses, bunyaviruses, arenaviruses, picornaviruses, filoviruses and also against SARS/MERS-CoVs.
Chloroquine
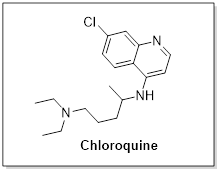
In history of past 60 years Chloroquine has been used globally as anti-malarial and immunomodulant drug. Chloroquine is an orally administered drug which has well known pharmacokinetics. WHO has put chloroquine in list of essential medicines. Recent studies reveal its efficacy in reducing viral replication including MERS-CoV and SARS-CoV hence, a broad spectrum antiviral therapeutic. Established clinical safety and economic reliability highlights its repurpose potential in this pandemic situation.
Blocking of virus infection is done by Chloroquine as it is accounting into by increasing the pH of endosomes, required for virus/ cell fusion and obstructing the glycosylation process of cellular receptors of SARS-CoV. Functioning of chloroquine is demonstrated at both entry and at stage of post entry in Vero E6 cells of 2019-nCoV infection. Immune-modulating activity of chloroquine may also account in boosting its antiviral effect in vivo.
In vitro Studies
Chloroquine shows clinically achievable EC90 value against 2019-nCoV in Vero E6 cells i.e. 5.47 μM. Demonstration has been done on plasma of patient suffering rheumatoid arthritis who dosed with 500 mg administration.
Hydroxychloroquine
Hydroxychloroquine (HCQ), a derivative of CQ, was first synthesized in 1946 by introducing a hydroxyl group into CQ and was demonstrated to be much less (~40%) toxic than CQ in animals although mechanism of action both drugs is same i.e. endosomal acidification. HCQ is still widely available to treat autoimmune diseases, such as systemic lupus erythematosus and rheumatoid arthritis. Hydroxychloroquine (EC50=0.72 μM) was found to be more potent than chloroquine (EC50=5.47 μM) in vitro. Preliminary results of one study also suggest a synergistic effect of the combination of hydroxychloroquine and azithromycin. Azithromycin has been shown to be active in vitro against Zika and Ebola viruses and to prevent severe respiratory tract infections when administrated to patients suffering viral infection.
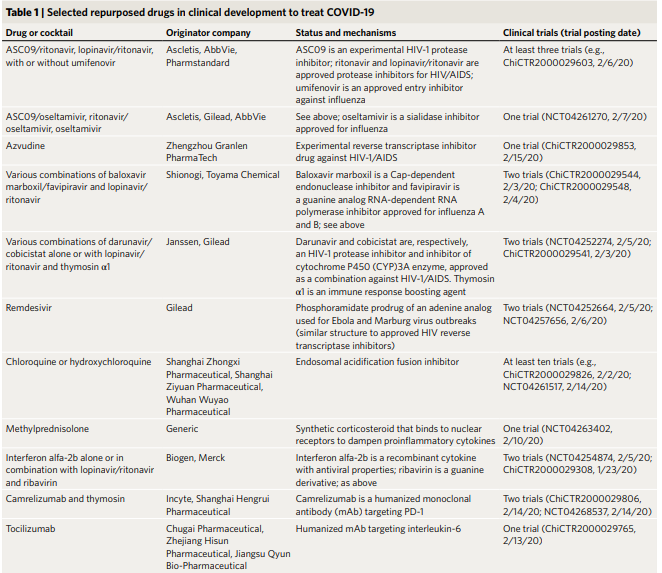
Table 1: Selected repurposed drugs in clinical development to treat COVID-19
Journal References
- Li G, De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV), Nature Reviews Drug Discovery 19, 149-150 (2020), https://www.nature.com/articles/d41573-020-00016-0
- Wang, M., Cao, R., Zhang, L. et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res 30, 269–271 (2020). https://doi.org/10.1038/s41422-020-0282-0
- Choy KT, Wong AY, Kaewpreedee P, et al. Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro [published online ahead of print, 2020 Apr 3]. Antiviral Res. 2020;178:104786. https://doi.org/10.1016/j.antiviral.2020.104786
- Nature Biotechnology, News 27 February 2020, https://www.nature.com/articles/d41587-020-00003-1
